How do Stem Cells Work?
The chart below shows the proven scientific ways that stem cells can improve tissue function. In laymen terms, we use different types of stem cells to assist different problems in the body. Sometimes the tissue needs increased blood supply and we give specialized cells called endothelial cell which stimulates a new vascular supply. Sometimes the cells need to be reprogrammed because, although the cells are alive, they are not functioning properly. As in the case of a stroke, the neural cells that we give actually reprogram non-functional cells around the damaged tissue caused by the stroke to again become functional. We use specific types of tissue stem cells to accomplish these various tasks, depending on the condition that we are treating. The nine different types of cells that we use, all heal using different mechanisms.
How are stem cells tested?
Over the years, regulatory agencies have developed specific criteria for testing the safety of tissues and stem cells for human use. This actually started with my associates in Moscow who have been making stem cells for over thirty-five years. The FDA, along with European Union regulators, has developed standards for the world and we adhere to these standards. The chart below shows the entire process of how the cells are tested.
Can I use my own cells or cells donated from someone in my family?
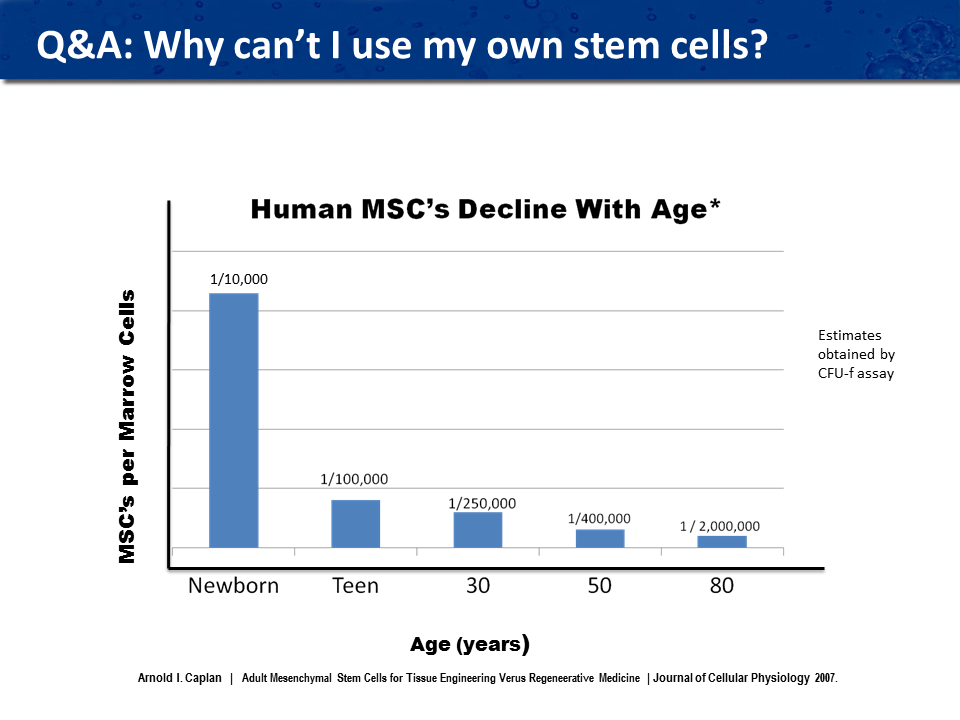
We do not use the patient’s own cells (autologous) or from a family member because we need to use cells from a younger source where there is a larger number of stem cells that are active. As you can see from the attached graph, in a newborn, one in ten thousand cells will be stem cells, while in a thirty year old, one in two hundred and fifty thousand cells will be stem cells, and in an eighty year old, only one in two million cells will be stem cells. Getting enough cells is now impossible as new FDA regulations forbid expanding autologous cells. All family member or donated cord blood cells would have to be tested using the FDA recommendations. Not to mention the expense of testing the cells, most of these cells donated in this fashion would not pass the strict cell testing criteria that that FDA has set.
Do I have to be type matched, like getting blood? Do I have to worry about my body rejecting the cells? Do I have to be on any immune suppressant drugs?
There is no cell typing necessary, when our cells are processed, there is no HLA (rejection proteins) on the cell surface. There is no problem with the body rejecting the stem cells and patients do not have to be on immune suppressant drugs. We have never had a patient experience adverse reaction from the cells. We have given some patients multiple stem cell treatments and there does not seem to be any build up of immune resistance to the cells.
How do I know I got the correct stem cells and enough cells?
I have taken great care in doing major research into all of the conditions that we treat. I believe a thorough understanding of the mechanism of the condition involved is required. I have researched both the pre-clinical (animal studies) tests that have been done and also, any stem cell or cell therapies that have been performed around the world. My Russian mentor, Dr. Mironov, experimented extensively on various dosage levels for each disease and I use as a foundation for current dosing of stem cells and selection of the appropriate stem cells to use in each condition. Most conditions requires two or more types of stem cells to accomplish the regeneration process. For example, in a stroke patient, we need to heal the damaged nerve tissue in the brain, and also have to address the vascular problem in the brain which was the initial of the stroke. Therefore, we use two different types of stem cell to accomplish. We have based the dosage of stem cells on the weight (size) of the patient and we also take into consideration the amount of damage.
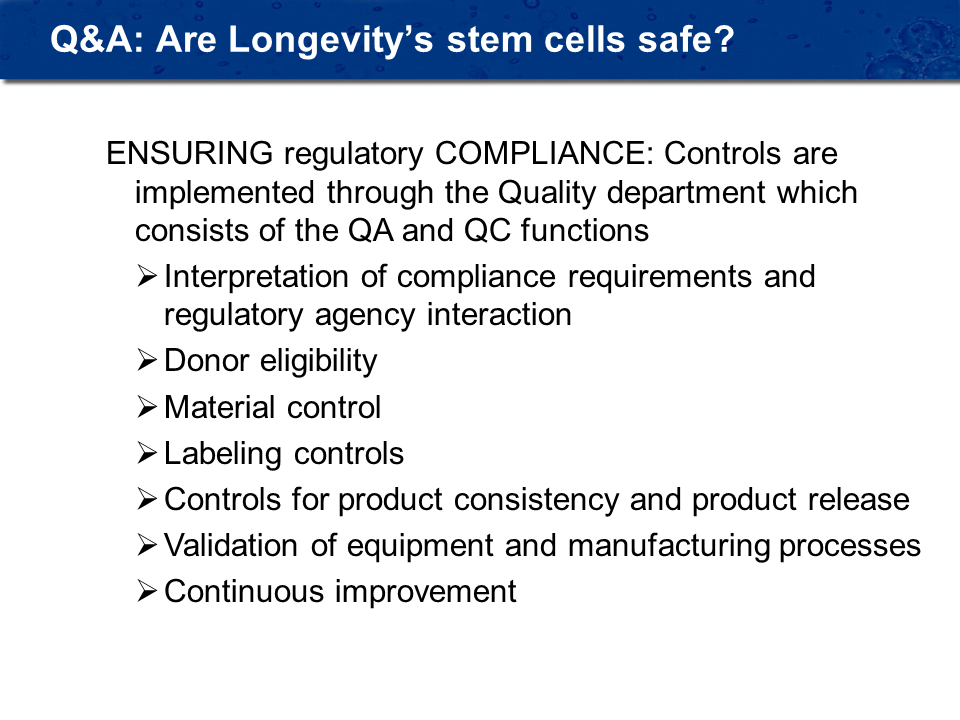
Are stem cells safe?
Yes, the donor is tested prior to tissue collection for viral & infectious diseases. There is karyotyping of cells for any chromosome abnormalities and tissue is tested for mycoplasma. Full safety, purity and potency testing is conducted. We have never had an adverse reaction to administration of the stem cells. See flow chart for stem cell testing protocol.
Why do I have to travel outside of the United States to get stem cell treatment?
The USFDA is on a very conservative pathway to approval for the use of stem cells in the United States. There currently are several stem cell clinical trials ongoing in the United States for various conditions. The criteria for entry into these clinical trials are very specific and very limited and these trials are only using one cell. It will be years before the U.S. is up to par in stem cell treatment using multiple types of stem cells to treat disease. For those individuals who are not willing to wait, or cannot wait, it will be necessary to travel outside of the United States to get this treatment.
Is there any situation in which an individual would not be able to get stem cell treatment?
Yes, we cannot administer stem cell treatment to anyone with an active cancer. These stem cells contain growth factors and it could be possible that the stem cells could activate and stimulate growth of a cancer. We are unsure of this theory, so this time we are taking a precaution in patients with active cancers or elevated tumor markers.
Is the stem cell treatment a complicated process?
No, stem cell treatment is a very simple procedure. Depending on the condition for which you are being treated, and what type of cells you will be getting, will depend on whether you even need to spend the night in the hospital. For those individuals who are being treated for a neurological problem (stroke, dementia, Alzheimer’s, Parkinson’s, spinal cord or traumatic brain injury, etc.), they will spend one night in the hospital. They will arrive at the hospital in the afternoon and the neural stem cells will be administered by an intrathecal injection (spinal tap). Afterwards, they will be monitored to track their vital signs for a few hours. The following morning, they will be given the additional cells intravenously which will take an hour, and then after a short period of time, they will be released back to their hotel. For those individuals who are not being treated for a neurological problem (heart, diabetes, liver, etc.), or who are getting and anti-aging protocol, they will get their stem cells intravenously and will only be at the hospital for a few hours.
How will I feel after I get these stem cells? Will I feel sick?
No, you will not feel sick after getting stem cells. After the mesenchymal stem cells are administered intravenously, some people feel tired that day and will want to take naps in the afternoon. But the following day, patients will not feel any residual tiredness.
How many stem cells will I be given?
The amount of stem cells you be given can vary depending on how many different types that are being administered, but normally patients will receive 75,000,000 to 100,000,000 mesenchymal cells and a similar number of neural and/or other different types of cells. The number of cells is dependent on the size of the patient, age, extent of the injury and other disease states which are present.
Can I get a stem cell treatment in Mexico?
The only cell that can legally be administered in Mexico is the mesenchymal stem cell for heart disease. For patients that have a heart problem and who are unwilling or unable to travel to Moscow, this is an alternative. It is not as good as going to Moscow where we have access to additional heart cells to augment the mesenchymal cells, but it is better than not getting stem cells as all. The Stemedica stem cells administered in Mexico have proven effective in clinical trials and therefore are an alternative for patients who cannot travel.
How soon after I receive the stem cells can I return home?
After receiving stem cells, patients can fly home the day, (24 hours) following the discharge from the hospital.
Will I need more than one treatment?
Sometimes more than one stem cell treatment is necessary to optimize therapeutic outcomes. For patients who are seeking stem cell treatment for cardiac problems or diabetes, frequently only one treatment may be necessary. For patients who have a neurological condition, patients may benefit from additional treatments. There has not been enough experience with stem cells to determine when we have achieved the maximum regenerative response.
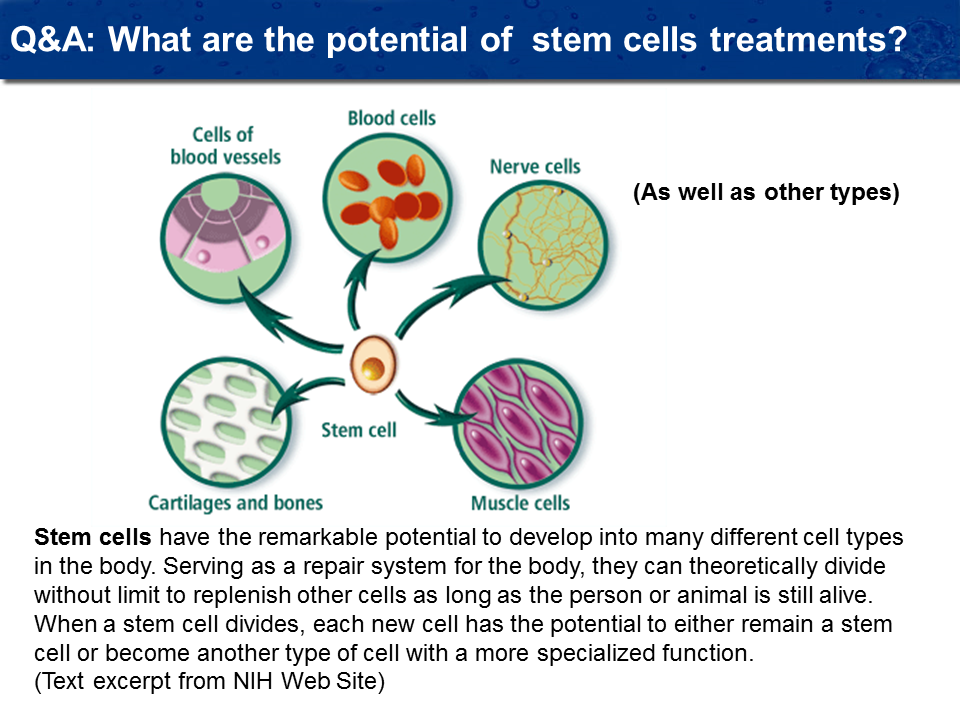
What are the types of stem cells?
Unlike any other place in the world that we know of, our lab can manufacture nine different types of stem cells. These are adult stem cells which are designed to heal specific tissues. Most researchers in the United States today have the availability of only one type of stem cell (a bone marrow Mesenchymal stem cell). There is some research beginning now using neural stem cells, but all of these projects are in the infancy stages. We both of these two types of stem cells, in addition to muscle stem cells, blood vascular stem cells, and specific neurological and organ stem cells, i.e. liver and heart muscle stem cells, to accomplish the healing process.
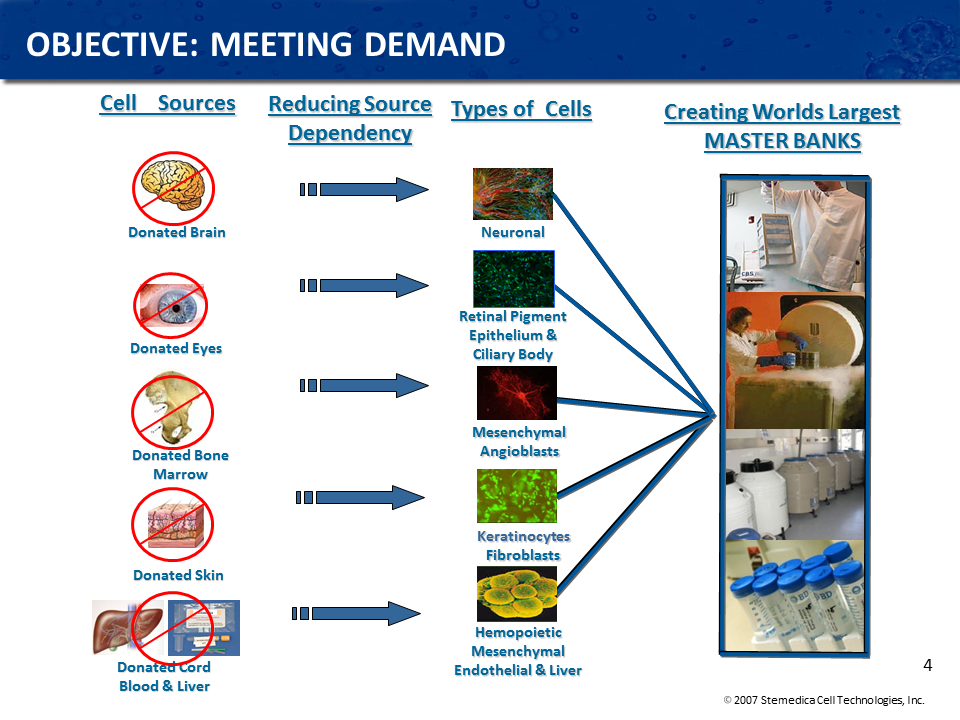
Where do the allogenic stem cells come from?
A stem cell donation program is very similar to any other organ donation program; in fact, stem cell donations must follow the same guidelines as an organ transplant. This is because allogeneic stem cells are all cultivated from donated organs. From donated brain tissue, for example, scientists can isolate neural stem cells which are essential in regenerating neurological conditions such as stroke, spinal cord and traumatic brain injuries. From bone marrow, they can derive mesenchymal stem cells as well as hematopoietic stem cells and endothelial progenitor cells, three distinct and potent cell lines. We have a variety of tissue sources, and many different cell lines to choose from so the treatment is more effective when the right types of cells are administered for the particular condition. While mesenchymal stem cells have a wide range of utility from a therapeutic standpoint, the stem cells of the heart, for example, are more effective for treating cardiac conditions. In addition to utilizing tissue from adult donors, fetal cells are also a particularly powerful stem cell source, where cells from the brain, eyes, liver, heart, and skin can be harvested. These fetal cells come from miscarriages or therapeutic abortions and in the same way that parents may donate their children’s organs, if the child passes away, organs from fetuses can also be donated. One fetal tissue donation can yield enough stem cells to treat thousands of potential patients. Other sources of allogeneic stem cells are cord, placenta and cord blood.
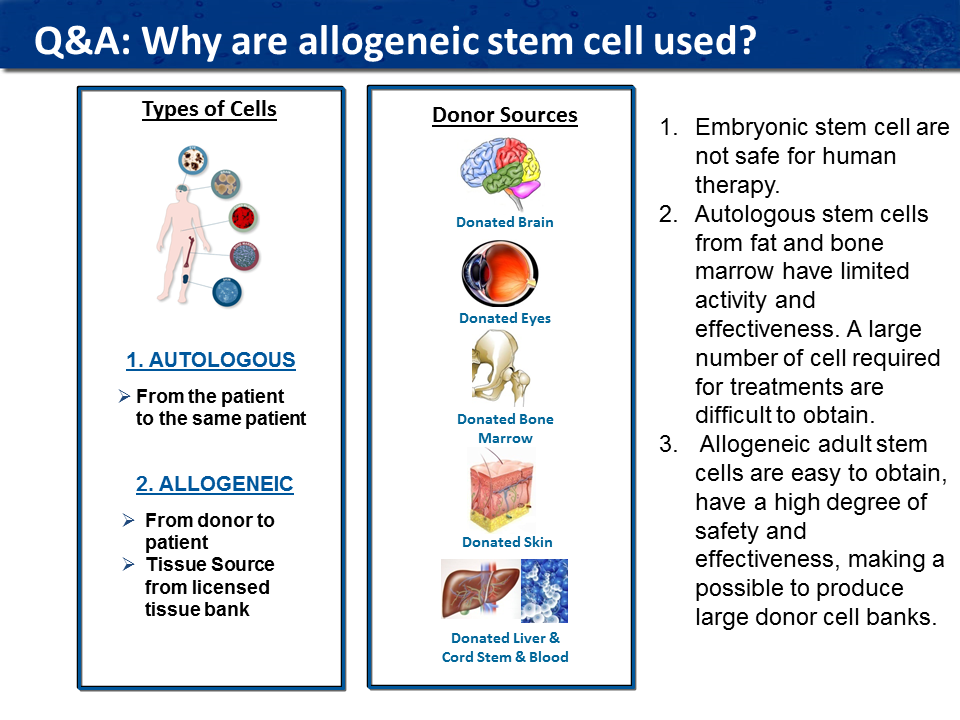
What are autologous stem cells?
Autologous stem cells are taken from the patient’s body and reinserted back into the patient. The major advantage to using autologous stem cells is that there is no FDA regulation preventing the doctors from using autologous stem cells. However, the major disadvantage is that they are cultivated from older or ill patients, which reduces the number, quality, and potency of the cells. As we age, it takes longer to heal from a wound because as we age, the number of stem cells in our body dramatically diminishes; they become weaker and not as active. Also, if the patient is being treated for a degenerative condition (Parkinson’s for example), then the autologous stem cells from bone marrow or adipose tissue cannot form neurological stem cells that are required for healing nerve tissue. For that reason, therapies using specific types of allogeneic stem cells provide a better alternative for a positive clinical response.
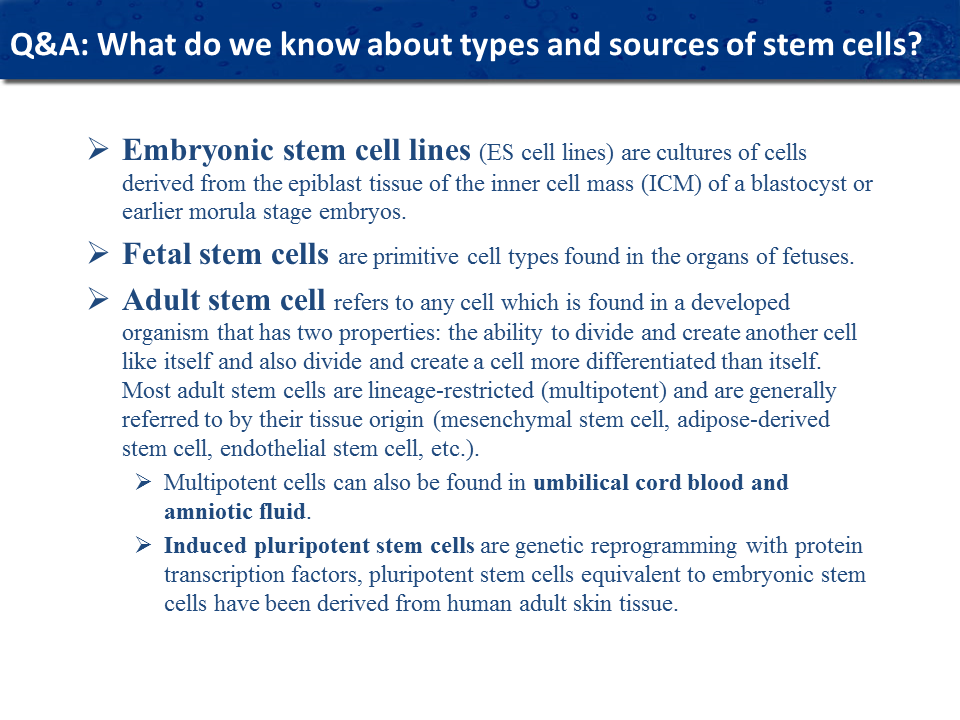
What are embryonic stem cells and do you use them?
Embryonic stem cells are cultivated from a four-day old fertilized human egg. The source for most of these embryonic stem cells is from embryos stored in fertilization clinics which are donated for scientific research by couples who decide not to use their embryos. Embryonic stem cells are now only used for research and are not used for treatment in humans because they are too potent and uncontrollable. Previously there were large instances where embryonic stem cells caused a type of tumor known as teratomas (Greek word for “monstrous tumor”). Embryonic stem cells are well suited for basic laboratory scientific research. Drugs can be tested using embryonic stem cells and the efficacy of the drug and its side effects can be determined before running a clinical trial.
What are allogenic stem cells?
Allogeneic stem cells are cells that are taken from a donor and administered to a patient. Allogenic stem cells are more active because they are often from significantly younger donors, and they also show more promise for treating degenerative diseases. Similarly, many diseases negatively affect the potency of a person’s own stem cells, (auto immune diseases) making the difference between autologous and allogeneic stem cells more pronounced. It is more efficient to create master banks of stem cells that have maximum potency and effectiveness and have been tested for safety. Research shows that the number of active molecules in allogeneic stem cells is much superior to autologous cells which are taken from the patient.